At Actulligence, we are deeply convinced that business intelligence should not be a burden on any organization. It should rely on the organization’s existing processes to answer operational and business needs and be integrated depending on how the user operates.
The usual cliché of business intelligence is the « intelligence cycle », as defined by Henri Martre who was one of the first to formalize the concept. The intelligence cycle has often been implemented, as much as commented, amended, criticized… but as far as I am concerned, I think that it’s a good starting point: it involves the essential steps enabling a virtuous information process. It is obvious that the intelligence cycle should never be applied independently from the existing processes, be they related to business, projects, or information.
That’s true as far as strategic, competitive, and technologic information are concerned, but that should be also true for social media information generated by a social listening process.
However, I can only regret that the multiple conferences I attend only focus on the communications and marketing side, with limited real-life examples and limited to « influencer monitoring » (involving the assessment of the influencer process ROI), the monitoring of gained benefits from a campaign (and assessment of the ROI of the services sold by the communications agency), the “share of voices” (who’s got the main share of voices).
I exaggerate a bit, because sometimes, in a burst of imagination, people raise their hands and point out the « voice of customers » (because customers also have ideas or because they are not happy: jump directly to the after-sales service.)
5 or 6 years ago, I had the opportunity to reflect on the best way to implement a social listening solution for the pharma industry, which is subjected to dire regulatory and communication constraints, as well as listening issues. Listening to the voice of consumers/patients, key opinion leaders, or health practitioners did not necessarily go without saying..
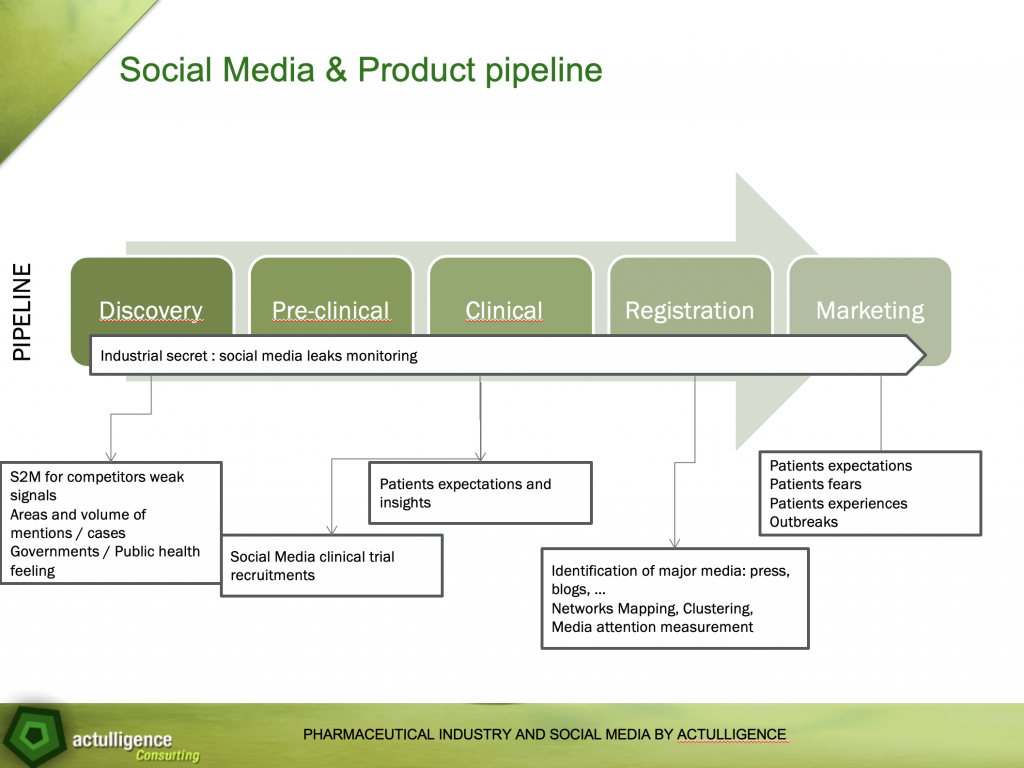
This work led me to combine social listening tools and the product life cycle (more precisely, a part of the product life cycle from fundamental research to marketing, I dropped the loss of exclusivity step).
We can summarize the drug development cycle in a few simplified steps:
- The discovery phase: the discovery of a molecule, an active ingredient, a new therapeutic axis for molecules, biologics
- The pre-clinical phase: before the clinical trials
- The clinical trials phase, during which a drug is assessed in terms of efficiency on patients
- The registration phase during which the drug is registered at national health authorities before receiving a market authorization
- The market phase during which the drug is available for patients.
How social listening can be applied? What is it going to bring and to whom during these phases?
We have identified several leads.

During the discovery phase, we can use social media to detect weak signals from competitors. What are the Twitter accounts that relay them? What are the interactions on LinkedIn? Do they retweet messages from other labs or are they focused on certain diseases? Let’s remain realistic. We are not likely to find 10-year development projects from our competitors, but analyzing some data, particularly interaction or word convergence, the results can sometimes be meaningful.
Likewise, during the discovery phase, it is possible to identify and assess the number of patients impacted or potentially concerned. No specific data, for sure. But the identification of countries, geographic zones in countries, and seasonality prove to be of interest.
As health authorities and governments are key stakeholders in the decision to reimburse a drug or not, their standpoints on a disease, their concerns can enable us to understand what are the countries likely to be interested in allowing the development of a new drug, and what are the stakes in terms of public health.
During clinical trials, one of the costliest points is patient recruitment: i.e. identifying patients wishing to participate in clinical trials for a new drug. While on « common » diseases, recruitment is easier, rarer diseases tend to have difficulties in recruiting patients that have the right criteria and ensure an objective assessment of the drug’s efficiency. Social media listening on forums, patient communities, or individual accounts, can facilitate this recruitment phase.
During clinical trials, patients’ expectations can also be identified. We are then able to go deeper on the analysis and understand the expectations in terms of quality of life, easiness to stick to a treatment, packaging issues (user-friendliness, readability, security, conservation), inconvenient side effects from existing treatments for the same pathology. These expectations can be integrated to clinical trials criteria.
The registration phase is a favorable ground to prepare the market launch. What are the press releases websites that are the most quoted in health media and by patients’ communities or individual patients? What are the existing communities, their main relays and players? Understanding how social and traditional media, patients, interactions work allows to convey the message to the right people.
During the marketing phase, after the marketing authorization, we can collect patient feedback on their treatment : perception of efficiency, decrease or disappearance of their symptoms and, although this is taboo in the heavily regulated pharma industry, detection of unidentified side effects before clinical trials (AKA pharmacovigilance for those in the know).
And finally, all along the process, implementing a long-term routine to spot internal potential information and industrial secrets leakages is not to be set aside. Videos, Slideshare presentations, Linkedin CVs giving too much details on a project… All these aspects of social listening are crucial for pharma or any other industry, but often forsaken.
Various pathways are thus open to us to apply social listening in a process involving conception and product launch. Social listening is not supposed to be restricted to communications teams’ needs, but instead has to find its place among other steps and internal clients.
You will find below a recapitulative diagram outlining this reflection, I found that sharing it with you would be of interest.

It is a stance we try to implement at Actulligence. Need for info exist and is widespread in a company. Instead of limiting ourselves in a thematic vision, we incentivize our clients to adopt a functional approach: what information do you need to make your decisions? Can social listening can answer this need?
We are deeply committed to delivering information where the users are. We limit the creation of new platforms in which the info is consulted and try to integrate it in existing spots such as project user groups and corporate social networks.
NB : it is relevant to stress that GDPR strongly narrows the use of personal data. As such, the above recommendations should be implemented with a cautious anonymization of the data collected and subjected to the right declarations.
NB2: integrated social listening solutions are increasingly having trouble in providing a relevant and efficient access to data, because of API restrictions from the main social networks. Agility will thus prevail in the implementation of social listening tools to answer this kind of needs.




