When it comes to competitive intelligence in the pharmaceutical sector, one of the key areas to monitor is clinical trials conducted by competitors.
One of the best-known and most visible websites in the world is the U.S. Food and Drug Administration’s www.clinicaltrials.gov
NB: This article refers to the new version of the clinicaltrials.gov website, which has recently changed. The old version and interface still exist on classic.clinicaltrials.gov and some of its functions are still useful for our purposes. We will point this out where applicable.
What are the best ways to monitor clinical trials run by pharmaceutical companies? Tips and tricks to make your life easier.
We hope you’ll find the following tutorial useful.
How to configure your monitoring
One of the great advantages of clinicaltrials.gov is that it has an RSS feed for searches that you can do on the website, making it much easier to monitor search results, both for new clinical trials and changes to existing ones.
The search functionality on clinicaltrials.gov is very comprehensive and offers a broad range of parameters.
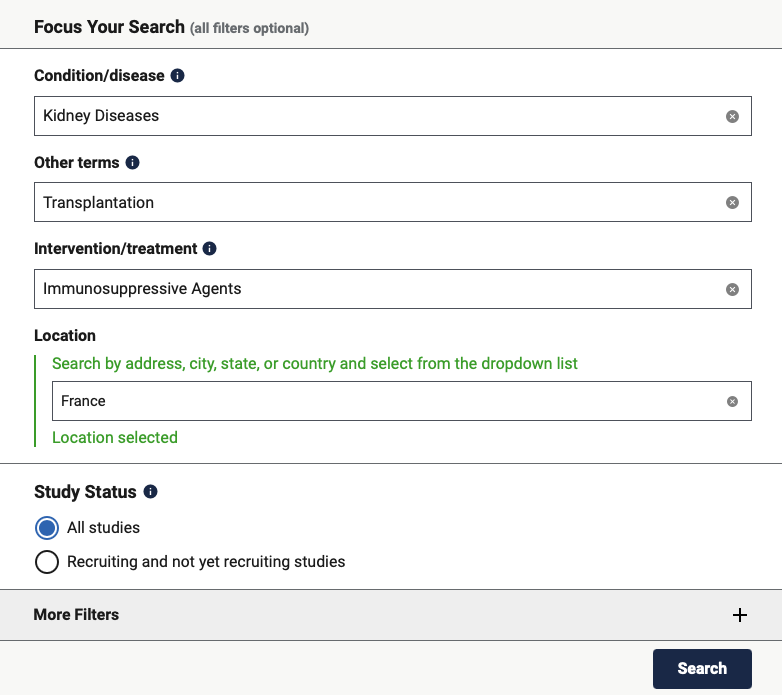
Simple search form on clinicaltrials.gov
This website provides a simple default search interface with just 5 fields:
- Condition/disease:
- You can target clinical trials for certain diseases or conditions. This field is useful when you want to monitor a particular therapeutic area and avoid the noise that comes with a free text search. For example, if you are looking for clinical trials related to diabetes but you type ‘diabetes’ in the ‘Other terms’ field, you will get treatments aimed at diabetics but also clinical trials that exclude diabetics. It is therefore strongly recommended that you use the ‘Condition/disease’ field when monitoring a specific therapeutic area. Note that as soon as you start typing, the search form will suggest therapeutic areas that it recognises and you then need to select the relevant therapeutic area.
- Other terms:
- This is a free text search that can be used either on its own or to help target the search in addition to the specific fields. For example, you can add the reference number of a particular clinical trial or the name of the company conducting it, etc. Numerous additional fields are available and the clinical trial number can also be entered in the ‘Study IDs’ field, for example.
- This search field allows advanced searches based on Boolean operators.
- To search with a character string consisting of several words, remember to use quotation marks. You can also use NOT to exclude certain words, e.g., influenza NOT seasonal.
- And finally, you can also use the operator AND to ask for two words in the clinical study to be displayed simultaneously, e.g., coronavirus AND influenza.
- Intervention/treatment:
- Intervention/treatment allows you to define what type of treatment or intervention will be carried out. For example, it could be a vaccine, radiotherapy, diet, etc.
- This field is not standardised and companies reporting clinical trials use it in different ways. You are advised to proceed with caution when using it, as you could miss new potential clinical studies.
- Location:
- This field is used to find out where a clinical study is taking place. Once again, the search form provides autocomplete based on recognised locations. This field can therefore be used to identify a clinical study centre used for a specific purpose, for example. For major products, phase III clinical trials are often carried out in a number of countries because some national regulations require drugs to be tested in a centre in that country before it can be approved there. Monitoring locations also provides clues as to which countries are being targeted by the company making the product.
- The ‘Facility name’ field in the advanced search form can also be used.
- Study Status:
- Study status is used to find out whether the clinical trial:
- Has not yet started recruiting patients (Not yet recruiting)
- Is currently recruiting patients (Recruiting)
- Recruits pre-selected patients and is not open to any patients other than those selected by the researchers running the clinical study (Enrolling by invitation)
- Has started and some patients are already receiving treatment but no new participants are being recruited. (Active, not recruiting)
- The study is currently suspended but might resume. (Suspended)
- The study has been stopped before the planned time and will not start again. No participant is receiving treatment or being examined. (Terminated)
- The study finished as planned and patients are no longer receiving treatment or being examined. (Completed)
- The study was ended before recruiting its first patient. (Withdrawn)
- The scheduled completion date for the study has passed and the last audit of this particular clinical trial was more than two years ago. Clinicaltrials.gov therefore considers the study has no known status. (Unknown)
- Study status is used to find out whether the clinical trial:
The advanced search form can also be used to enter many other fields to carry out a specific search of your choice using the most relevant fields.
Age ranges are used to find out if the treatment is intended for elderly people, pediatry or adults between the ages of 18 and 64. This field allows you, for example, to pinpoint treatments that have already obtained approval and are seeking to extend the authorised prescription to other age ranges.
The clinical trial phase (Study phase) is used to find out if the clinical trial is in an early phase (Phase 1) or the final phrase before potential approval (Phase III). Phase IV is a clinical trial for an already approved treatment.
The type of clinical trial (Study type) is used to determine whether it is an interventional study, i.e., an actual clinical trial, or an observational study, i.e., a clinical study in which the patients are identified and may have received treatments or interventions but not through the person conducting the study. Observational studies can be used, for example, to pool the results of different clinical trials and draw broader conclusions about a particular treatment or intervention. Finally, open studies (Expanded access) are open to patients with diseases or conditions that do not yet have any effective approved treatment and who may be given the go-ahead to participate in the relevant studies.
The filter field related to the availability of results is important because it can be used to make specific queries once clinical trial results are published. It should be noted that the results are often available elsewhere before they are published on clinicaltrials.gov (for example, in scientific journals). However, since 2021, the FDA has been much stricter about the publication of results than it was in the past and the results are now more easily accessible via this website. But despite increasing pressure from the FDA to publish results, and despite the obligation on researchers to publish within a year (requirement in force since 2017), many companies still do not publish and the FDA still does not appear to be issuing any fines.
The ‘sponsor or collaborator’ and ‘sponsor (Lead)’ fields are used to identify a company or organisation that is conducting or involved in a clinical trial. This is especially useful for monitoring a specific company.
The ‘Study IDs’ field is used to focus on one particular study. This field takes into account the clinical trial identifiers from the clinicaltrials.gov database, but can also be used to pick out identifiers from other databases that have also been reported on clinicaltrials.gov
How to generate an RSS feed
To make the most of an RSS feed, you have to first type in your search and view the results. The RSS feed can then be personalised in a very precise way, based on all your search parameters.
Here is an example:
- Studies on disorders related to transplants (conditions/diseases: Transplant-Related Disorder)
- Phase III
- Completed
- With results
By monitoring this query, we can identify, for example, all studies that might publish results in the future.
RSS feeds are generated using the icon on the top right of the results list.
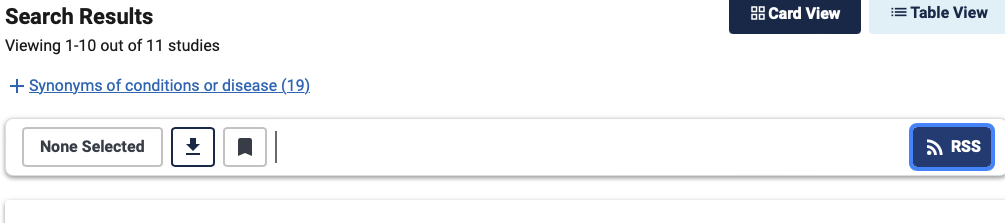
Click on it and a window appears that will allow you to select the RSS feeds you want.
There are two types:
- An RSS feed to identify all clinical studies newly registered on the CT. gov website. In this type of search, the RSS thread will not be very useful. However, the RSS feed for new clinical studies can be used to identify a new clinical trial in a specific therapeutic area or company as soon as it is published. You can then monitor the clinical study page directly to be kept informed when changes occur. By default, this RSS monitoring thread covers the last fourteen-day period.
- An RSS feed that identifies new clinical studies or any changes to them over the past 14 days. In our example, you need to apply this RSS feed as it will enable you to identify the publication of the results of a study even if it was registered on the CT. gov site a long time ago.
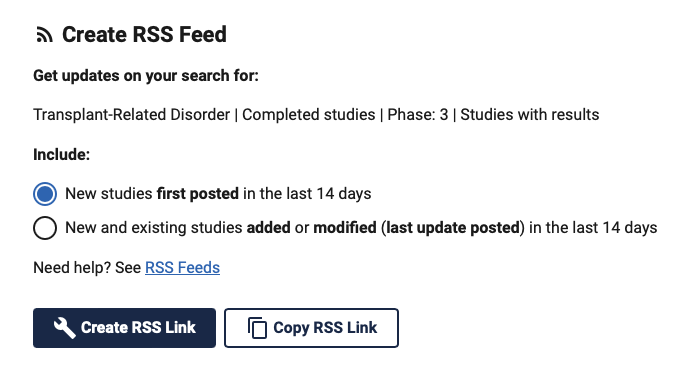
Once you have made your choice, you have two options:
- Create RSS feed: when you click on this option, your computer will try to find an RSS reader by default and add the RSS thread to it.
- Copy RSS link: the RSS feed link is copied to your clipboard and it is then up to you to add it to the RSS app of your choice or to your monitoring software.
To continue the monitoring, you need to configure your RSS reader correctly to be informed of any updates to the RSS feeds.
There are many options you may wish to consider.
If you use Inoreader, you can, for example, use automatic tagging rules and internal notifications in the app as your basis or you can use IFTTT to link it to Inoreader and set up advanced sharing and distribution rules.
If you use monitoring software, you can take it further and, for example, retrieve the entire page related to the clinical study rather than just an update or availability notification.
The main limitations of RSS feeds on clinicaltrials.gov
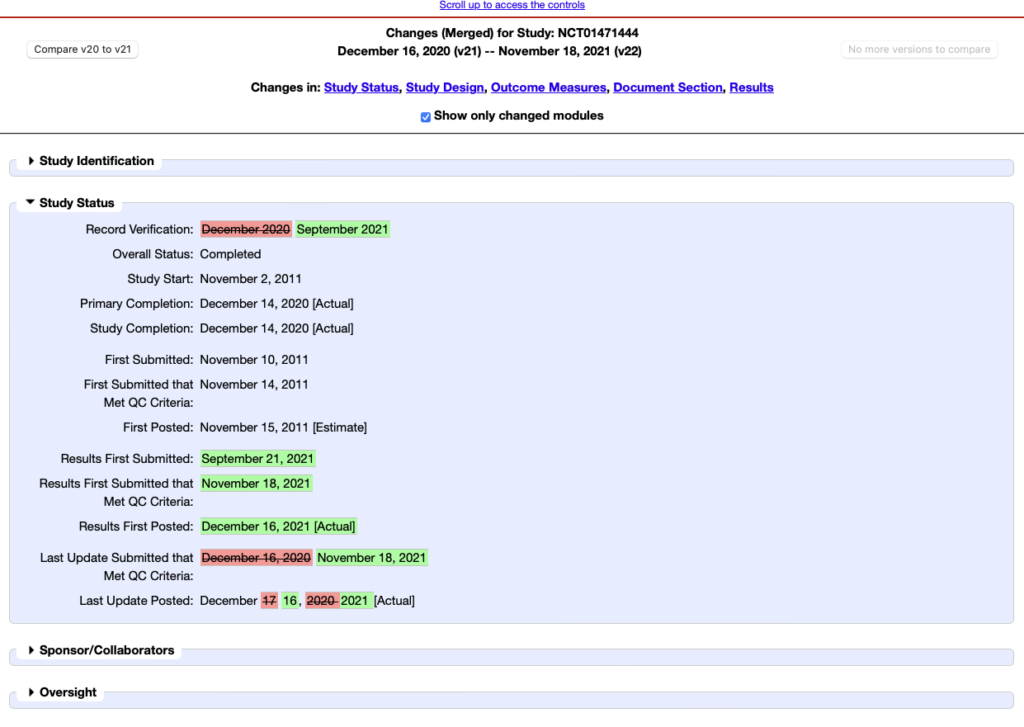
One of the main difficulties in monitoring clinical trials is identifying changes that have occurred in the trial.
When you view a clinical study via the RSS feed, you are directly accessing the latest version and the changes are not marked. For example, the RSS feed can tell you if the study status has changed but it is up to you to go and check the previous status using the version comparison.
To do that, you need to scroll down to the bottom of the clinical study page and click on ‘Record history’. You can then choose which versions of the clinical study you wish to monitor and view the differences. The problem is that this can be a bit fiddly.
If you want to keep track of all the changes in a clinical study, monitoring each clinical study directly using monitoring software that copies the original page and displays the changes directly is likely to prove more effective.
Most professional monitoring software has these page monitoring and change notification functions and you can always use less expensive software such as the Update scanner plugin or the Saas solutions, distill.io or changedetection.io.
There are many options but it is worth noting that some software can fail to effectively monitor a clinical study either because the HTML page layout is based on tables, or because the website itself prohibits access to certain crawlers.
Another limitation of RSS feeds for monitoring clinical studies relates to the publication of results. You often receive notification of a change to a clinical study and when you look at the ‘Record history’, the latest version of the clinical study pre-dates the notification of the last change. This often happens when results are published and available several days after notification. It is then imperative to return to the clinical study at a later date to check the results.
- Other clinical trials websites
While the clinicaltrials.gov website is essential for monitoring the competition in the pharmaceutical sector, other countries also keep databases of clinical trials.
The WHO website has an exhaustive list of these databases on the two pages below, one for primary suppliers and the other for partners:
NB: the clinical studies website provided by the WHO incorporates a large number of local clinical trials databases, which is helpful and can be used to centralise monitoring rather than monitoring several different registries. However, the data is sometimes uploaded to the WHO metasearch engine very late, which, in our opinion, hampers performance and responsiveness for a monitoring project. Furthermore, the WHO website does not provide such detailed search options in terms of fields, nor does it provide RSS.
Actulligence specialises in the implementation of monitoring projects and also undertakes strategic monitoring and monitoring of competitors on a daily basis for its clients. We are able to work with most professional monitoring software on the market, and can configure it and maintain connection to the sources. We work with both simple and complex sources to help our clients identify useful information as quickly as possible. We work with customised projects so you can be sure that the most useful sources for you are fed into your strategic vision every day.




